With the advancement of cognitive neuroscience and the development of modern brain imaging methods in the 1990s, the field of language neurobiology—the study of the relationship between the brain and language functions—has grown immensely and rapidly over the past thirty years, and finds itself at a crossroad with respect to its theoretical underpinning. The main message of our recent article “Broca and Wernicke are Dead, or Moving Past the Classic Model of Language Neurobiology” (Tremblay & Dick, 2016) is that the most historically important model in the field, the Classic “Wernicke-Geschwind” Model, and associated terminology, is no longer useful to guide research and clinical intervention in the field.
A Brief History of the Classic Model
The Classic Model was the first major model of language neurobiology. It has dominated the field of language neurobiology for over 150 years now, guiding both research and clinical interventions and influencing many generations of scientists. The Model was first introduced in Europe in 1861 and in 1874 as a response to an argument about how the brain works that was prevalent in the nineteenth century. At that time, several scientists were opposed to the notion that specific areas of the brain had distinct functions, arguing instead that the brain-as-a-whole worked as one functional unit. This meant that a lesion to one part of the brain could be accommodated by the rest of the brain. Other scientists, often referred to as the localizationists, were convinced that that brain was organized into several functional units and that lesion to one unit would lead to the loss of the specific function served by that unit. This was referred to as the cortical localization of functions.
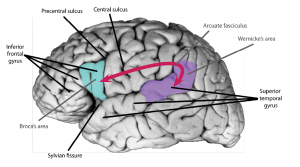
Though several scientists have contributed to the elaboration of the Classic Model, the contribution of two pioneer physicians is particularly important: the French Pierre Paul Broca (1824–1880), and the German Carl Wernicke (1848–1905). In the 1860s, Broca presented the results of a series of autopsies of patients with difficulties producing language. His analysis revealed localized damage to the left frontal lobes in the perisylvian area, that is, close to the Sylvian fissure (Figure 1). These findings were interpreted as evidence in favour of the cortical localization of function. They were also interpreted as some of the first scientific evidences that language is lateralized to the left hemisphere, a phenomenon that is often referred to as cerebral dominance. A few years later, Wernicke presented evidence that damage to the posterior part of the left superior temporal lobe (the so-called “Wernicke’s area”) is associated with difficulty understanding language, further supporting the notions of cortical localization and cortical dominance. Wernicke, and later Ludwig Lichtheim (1845–1928), also a German physician, elaborated the firsts neurobiological account of human language, laying the foundations of the Classic Model, often referred to as the Wernicke-Lichtheim Model, or sometimes as the Broca-Wernicke Model given the important discoveries made by the two physicians. The model continued to evolve to explain different clinical symptoms. Over one hundred years later, the model was updated and popularized by the American neurologist Norman Geschwind (Geschwind, 1970) to the model presented in Figure 1. This version of the Classic Model is the one that most students are taught in their introductory psychology, linguistics, neuroscience, or medical school textbooks.
Overview of the Criticisms of the Classic Model
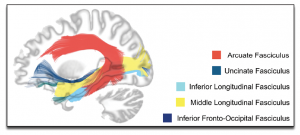
The work of pioneer scientists such as Broca, Wernicke and Lichtheim has had a major impact on our understanding of brain/behaviour relationships, and it has influenced the work of several generations of scientists. Yet, 150 years later, and with our modern knowledge of brain anatomy, we argue that the Classic Model suffers from two main issues: (1) it is centred on two “language regions” and one fibre pathway (the arcuate fasciculus; see Figure 3), and (2) the precision of the model is too limited to test specific hypotheses about brain/language relationships. Our main take-home message is that keeping alive a model so outdated has a detrimental impact on research in the field of the neurobiology of language and has presented an oversimplified model of language neurobiology to the public. This is why we argue in favour of abandoning the model and associated terminology. In the next section, we will lay the foundations for these claims.
Criticism 1: Simplicity of the Model
The field of language neurobiology originates from the study of brain lesions in patients with language disorders. Lesion studies have had a major impact in shaping the field. But the advent of modern brain imaging techniques in the 21st century (See Box 1) has revolutionized the way that we approach the human brain and what we know about brain/language relationships.
|
Box 1. A history of modern imaging techniques in 6 important dates
|
An important take-home message is that all available scientific evidence suggests that language is not controlled by a network as simple as the one portrayed by the Classic Model. Language is a remarkable, human-specific faculty that allows us to express the most complex ideas and the deepest thoughts using long complicated strings of co-articulated speech sounds in just few seconds! The adult human is capable of producing six to nine speech sounds per seconds! The human brain is also capable of processing these sounds very quickly, and is able to access to the meaning that is carried by the sounds and the order in which the sounds are presented. Language can therefore be seen as one of the most complex cognitive and sensorimotor acts that the human brain can accomplish. That level of complexity requires a heavy machinery behind it to secure it, and multiple systems are involved including the auditory and visual systems, to the motor system and several cognitive networks including some that are language-specialized and others that are domain-general such as the attention networks.
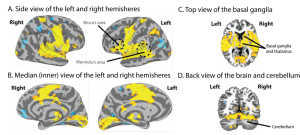
Thanks to the advent of powerful brain imaging techniques, evidence now abounds that regions far beyond the Classic language centres are involved at all levels: from sound perception, to comprehension, to the elaboration of complex messages and finally the production of speech sounds. As you can see from Figure 2, which is taken from a recent study from our group (Tremblay, Deschamps, Baroni, & Hasson, 2016), large sections of the brain, shown in yellow, are active even for a very simple task. These regions extend far beyond classical language regions which are represented using dotted lines in the figure. Importantly, as can be seen in the figure, language is supported by brain regions outside the frontal and temporal lobes—language is distributed across the cortex and it includes subcortical regions such as the basal ganglia, thalamus (Figure 2C), and cerebellum (Figure 2D). Another important take-home message is that the activity that is seen spans across both hemispheres and not just the left one. This does not mean that the left hemisphere is not involved, but it means that the right hemisphere participates as well, in many different ways. This and hundreds of studies like this one are very powerful demonstrations that the Classic Model is no longer an adequate model for explaining brain-language relationships and to guide research in the field.
Furthermore, contrary to the Classical Model, evidence suggests that there is not just one white matter tract—the arcuate fasciculus—that supports the language system. There are in fact many tracts, including the arcuate fasciculus, that support these functions (both production and comprehension; (for a review, see Dick, Bernal, & Tremblay, 2014; Dick & Tremblay, 2012). Recent advances in neuroimaging methods have led to the identification of many fibre pathways, and studies have found that the size or integrity of many of these tracts is correlated with speech and language performance, including the uncinate fasciculus, the inferior fronto-occipital fasciculus, the inferior longitudinal fasciculus, and the middle longitudinal fasciculus (Figure 3) This stands in direct contrast to almost every textbook presentation of how the brain processes language, as these typically focus on the two-region, one connection framework of the Classic Model. But this is what we have learned from over 30 years of brain imaging, and it is very well established in the scientific literature.
Criticism 2: Lack of Precision
Another important argument in our previous article is that there is also an important problem with the precision of the terminology associated with the Classic Model. The issue is that neither Wernicke’s nor Broca’s area are regions of the brain that are clearly defined. In our original article, we conducted a survey to demonstrate this point. We asked 159 people working in the field to identify which of a series of visual representations of Broca’s and Wernicke’s areas they think was correct. The majority of the respondents (87%) reported working in an academic setting, and 11% reported working in a clinical or hospital settings. 73% held a PhD; 13% a master’s degree; 9% a medical degree and 4% a baccalaureate. Respondents worked in a variety of disciplines (speech and language pathology, psychology, neurology, & linguistics), with an average of nine years of experience. This shows that our respondents were qualified to undertake this survey. Essentially supporting our argument, none of our representations of Wernicke’s areas garnered more than 30% of the votes. For Broca’s, none of our definitions garnered more than 50%. What these findings demonstrate is that experts in the field use the same words to refer to different things. This is a problem because to understand the precise functional organization of the brain, we need to be very precise. We need to have clear definitions that are agreed upon. This is why we recommend using an anatomical terminology. The words Broca’s area and Wernicke’s area are not anatomical labels, but functional ones. For example, Broca’s area refers to part of a region that is called the inferior frontal gyrus or IFG. As we already mentioned, the issue is that different scientists use the same word to refer to different locations within this general area. The IFG contains three divisions (opercular, triangular, & orbital). The definition of the IFG and each of its divisions are clear and agreed upon. Thus, when scientists report activation in the IFG in a scientific article or during an oral presentation, we recommend that they use the label IFG, pars opercularis or IFG, pars triangularis, for example, because this is more specific than Broca’s area.
Anatomically, the regions of the brain typically referred to as Broca’s and Wernicke’s areas are large enough that they do not have architectural homogeneity (Box 2). This means that, at the microscopic level, the cellular organization of the neurons in these areas is not homogeneous. Importantly, anatomical heterogeneity is usually coupled with functional heterogeneity. What this means is that within the boundaries of both Broca’s and Wernicke’s area lies a web of regions with distinct organization and presumably different functions. For example, Wernicke’s area contains at least three different regions (named BA 39, 40 and 22, and often 21, 22 and 37 as well, see Box 2). Each of these regions is believed to play specific roles given its different cellular organization and connectivity. For instance, the regions labelled BA 41 and 42, which are referred to as primary auditory regions, are the first to receive the sounds, including speech sounds, in the cortex (the outer covering of the brain which contains the body of the neurons). This is why their functional name is primary auditory cortex. In contrast, surrounding BA 22 only receives speech inputs after they have been processed in BA 41 and 42. It often referred to as association auditory cortex.
|
Box 2. Brain architecture The study of the architecture of the cerebral cortex, which contains the bodies of the neurons (the gray matter), is called cytoarchitectonics. It focuses on parsing the brain to reveal how neurons are organized into layers. There are six main layers (numbered I to VI from the outermost to the inner most). Each layer contains a specific distribution of neuron types as well as specific connections with other cortical and subcortical regions. The organization of the layers varies significantly across the cortex. It is usually considered that a piece of cortex with a uniform organization serves a specific set of functions. For example, the organization of the auditory cortex is different from the organization of the motor and visual cortices. Different scientists have proposed classification methods for the different parts of the cortex. The German neurologist Korbinian Brodmann (1868 –1918) proposed one of the classification methods that is most commonly used to refer to different parts of the cortex (Brodmann, 1909, 2006). Brodmann identified 52 distinct regions in each hemisphere, known as the Brodmann areas or sometimes BA. For example, the motor cortex corresponds to BA 4 and the auditory cortex corresponds to BA 41 and 42. |
In order to guide research, it is important to employ a specific terminology, one that allows us to distinguish between all the sub regions. This is crucial to further current understanding of brain organization and brain/language relationships.
Conclusions
Language neurobiology is a field of intense research and one that is evolving very rapidly, away from the classical and simplistic explanations of the last century. Here we argue that the Classic Model is now too simplistic and too imprecise to be useful in guiding both research and clinical practice. Importantly, we believe that the continuous use of the Classic Model and associated terminology has hindered our progress towards understanding the complexity of brain/language relationship. The development of modern brain imaging tools has really helped scientists make important strides towards developing better models of language neurobiology. This opens the door to finally solving the many mysteries about language and language disorders with which the modern science has been struggling. These advances will undoubtedly lead to the development of new and more effective interventions to treat language disorders resulting from brain disease.
We also think that language research is extremely important. Language is the means through which we communicate our ideas, thoughts and feelings, teach, learn, and make contact with other people. This is a function that is central to social communication and learning and, as such, it deserves more attention from the scientific community, the public, and the media. As much as we have learned tremendously over the past 30 years, so much more learning is yet to come!
References
Brodmann, K. (1909). Vergleichende Lokalisationslehre der Gro hirnrinde. Leipzig: Verlag von Johann Ambrosius Barth.
Brodmann, K. (2006). Brodmann’s Localisation in the Cerebral Cortex (L. J. Garey, Trans.). New York: Springer.
Dick, A. S., Bernal, B., & Tremblay, P. (2014). The language connectome: new pathways, new concepts. Neuroscientist, 20(5), 453-467. doi:10.1177/1073858413513502
Dick, A. S., & Tremblay, P. (2012). Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain, 135(Pt 12), 3529-3550. doi:10.1093/brain/aws222
Geschwind, N. (1970). The organization of language and the brain. Science, 170, 940-944.
Tremblay, P., Deschamps, I., Baroni, M., & Hasson, U. (2016). Neural sensitivity to syllable frequency and mutual information in speech perception and production. Neuroimage, 136, 106-121. doi:10.1016/j.neuroimage.2016.05.018
Tremblay, P., & Dick, A. S. (2016). Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang, 162, 60-71. doi:10.1016/j.bandl.2016.08.004
1 Correspondence concerning this article should be addressed to Pascale Tremblay, Département de Réadaptation, Université Laval, Québec (QC), G1V 0A6, Canada.
Email: pascale.tremblay@fmed.ulaval.ca
Author Bios

Pascale Tremblay is associate professor in Rehabilitation (speech pathology) at Université Laval in Québec City, Canada, and the co-founder of the international Society for the Neurobiology of Language (www.neurolang.org)
Anthony Dick is associate professor of Psychology at Florida International University in Miami, USA.
